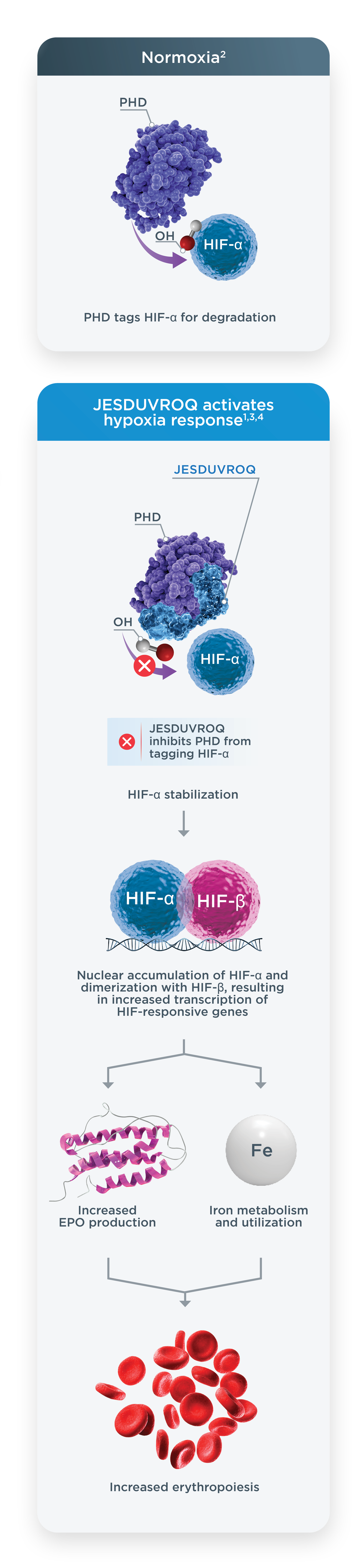
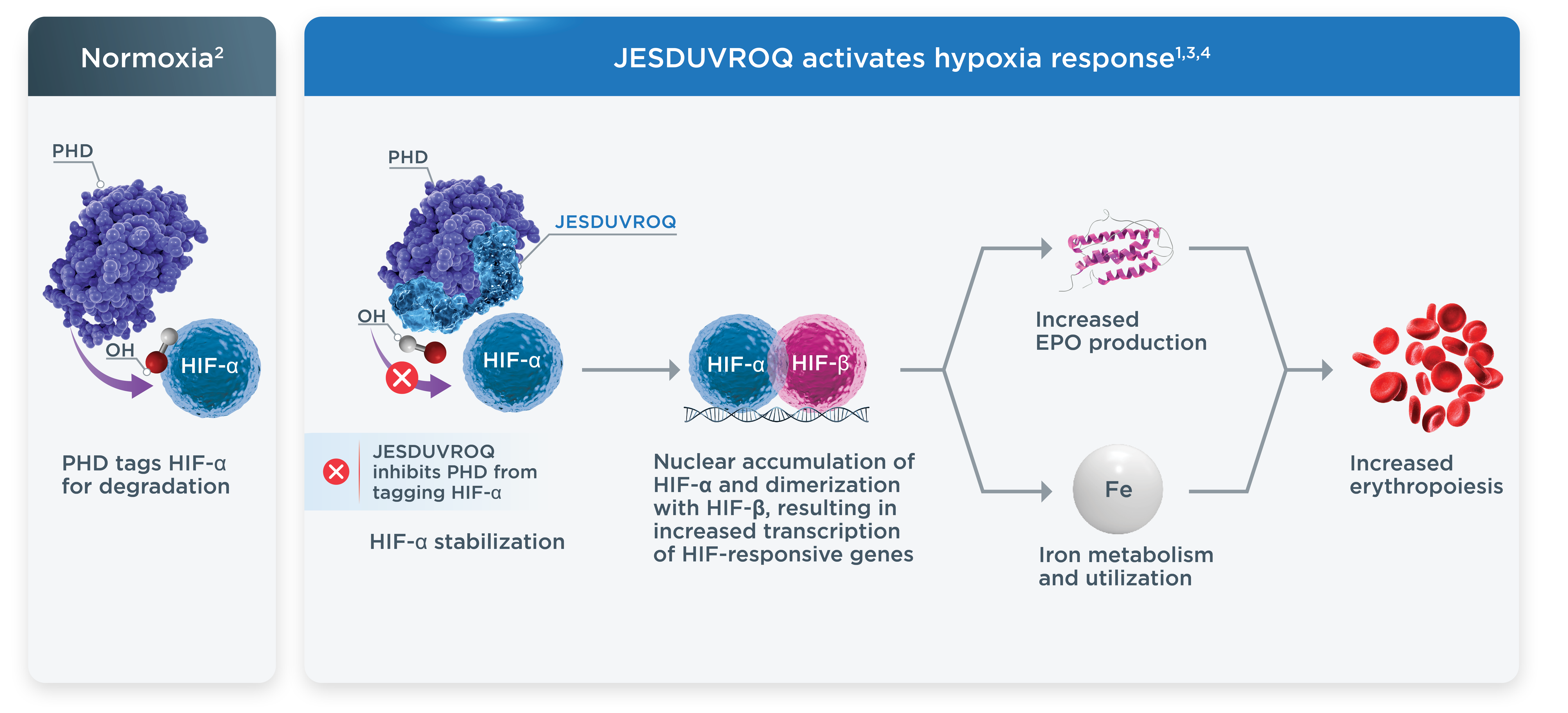
JESDUVROQ is indicated for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least four months.
Limitations of Use
JESDUVROQ has not been shown to improve quality of life, fatigue, or patient well-being.
JESDUVROQ is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or in patients who are not on dialysis.
JESDUVROQ is indicated for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least four months.
Limitations of Use
JESDUVROQ has not been shown to improve quality of life, fatigue, or patient well-being.
JESDUVROQ is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia or in patients who are not on dialysis.
JESDUVROQ is indicated for the treatment of anemia due to chronic kidney disease (CKD) in adults who have been receiving dialysis for at least four months.
Limitations of Use
JESDUVROQ has not been shown to improve quality of life, fatigue, or patient well-being.
JESDUVROQ is not indicated for use:
- As a substitute for red blood cell transfusions in patients who require immediate correction of anemia.
- For treatment of anemia of chronic kidney disease in patients who are not on dialysis.
WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, and THROMBOSIS OF VASCULAR ACCESS
- JESDUVROQ increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE).
WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, and THROMBOSIS OF VASCULAR ACCESS
- JESDUVROQ increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE).
- Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels.
- No trial has identified a hemoglobin target level, dose of JESDUVROQ, or dosing strategy that does not increase these risks.
WARNING: INCREASED RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, and THROMBOSIS OF VASCULAR ACCESS
- JESDUVROQ increases the risk of thrombotic vascular events, including major adverse cardiovascular events (MACE).
- Targeting a hemoglobin level greater than 11 g/dL is expected to further increase the risk of death and arterial venous thrombotic events, as occurs with erythropoietin stimulating agents (ESAs), which also increase erythropoietin levels.
- No trial has identified a hemoglobin target level, dose of JESDUVROQ, or dosing strategy that does not increase these risks.
- Use the lowest dose of JESDUVROQ sufficient to reduce the need for red blood cell transfusions.
CONTRAINDICATIONS
JESDUVROQ is contraindicated in patients:
- Receiving a strong CYP2C8 inhibitor such as gemfibrozil.
- With uncontrolled hypertension.
WARNINGS AND PRECAUTIONS
Increased Risk of Death, Myocardial Infarction, Stroke, Venous Thromboembolism, and Thrombosis of Vascular Access
JESDUVROQ increases the risk of arterial and venous thrombotic events, that may be fatal, including myocardial infarction, stroke, venous thromboembolism and vascular access thrombosis. Patients with cardiovascular or cerebrovascular disease are at increased risk of these events. Avoid use in patients with a history of myocardial infarction, cerebrovascular event, or acute coronary syndrome within the 3 months prior to starting JESDUVROQ.
A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks. Targeting a hemoglobin level of greater than 11 g/dL is expected to further increase the risk of death and arterial and venous thrombotic events, as occurs with ESAs, which also increase erythropoietin levels.
No trial has identified a hemoglobin target level, dose of JESDUVROQ, or dosing strategy that does not increase these risks. Use the lowest dose of JESDUVROQ sufficient to reduce the need for red blood transfusions. Adherence to dosing and hemoglobin monitoring recommendations is important to avoid excessive erythropoiesis.
Advise patients to seek immediate medical attention if they develop signs or symptoms of myocardial infarction, stroke, venous thromboembolism, or thrombosis of vascular access. Evaluate and manage promptly if these occur.
Risk of Hospitalization for Heart Failure
Hospitalization for heart failure was observed in 7.5% (3.3 per 100 Person Years [PY]) of patients on dialysis receiving JESDUVROQ and 6.8% (3.0 per 100 PY) of patients receiving recombinant human erythropoietin (rhEPO). Patients with a pre-existing history of heart failure were at increased risk of hospitalization for heart failure with JESDUVROQ (14.5%; 6.8 per 100 PY) compared to rhEPO (11.3%; 5.1 per 100 PY).
Consider the patient’s history of heart failure when deciding whether to prescribe JESDUVROQ. Advise patients of the symptoms and signs of heart failure and to immediately report any worsening to their healthcare provider.
Hypertension
JESDUVROQ is contraindicated in patients with uncontrolled hypertension. Worsening of hypertension occurred in 24% (12 per 100 PY) of patients receiving JESDUVROQ and 24% (12 per 100 PY) of patients receiving rhEPO. Serious worsening of hypertension occurred in 3.1% of patients receiving JESDUVROQ and 3.1% of patients receiving rhEPO. Cases of hypertensive crisis including hypertensive encephalopathy and seizures have also been reported in patients receiving JESDUVROQ. Monitor blood pressure and adjust or initiate anti-hypertensive therapy as needed.
Gastrointestinal Erosion
Gastric or esophageal erosions occurred in 5.7% (2.5 per 100 PY) of patients receiving JESDUVROQ and 6.6% (2.9 per 100 PY) of rhEPO-treated patients. Serious erosions, including gastrointestinal (GI) bleeding and the need for red blood cell transfusions, were reported in 3.6% and 3.1% of those receiving JESDUVROQ and rhEPO, respectively. Consider this risk particularly in patients at increased risk for GI erosions, such as those with a history of GI erosion, peptic ulcer disease, use of concomitant medications that increase the risk of GI erosion, and current tobacco smokers and alcohol drinkers.
Advise patients of the symptoms and signs of gastric and esophageal erosions and of gastrointestinal bleeding and to seek prompt medical care if these occur.
Serious Adverse Events in Patients with Anemia Due to Chronic Kidney Disease and Not on Dialysis
The safety of JESDUVROQ has not been established for the treatment of anemia due to CKD in adults not on dialysis and its use is not recommended in this setting.
In a large cardiovascular outcomes trial in adults with anemia of CKD who were not on dialysis, an increased risk of cardiovascular mortality, stroke, thromboembolism, serious acute kidney injury, hospitalization for heart failure, and serious GI erosions was observed in patients treated with JESDUVROQ compared to rhEPO.
Malignancy
Because increased hypoxia inducible factor (HIF)-1 levels may be associated with unfavorable effects on cancer growth, JESDUVROQ has not been studied and is not recommended in patients with active malignancies. Malignancies were observed in 4.4% (1.9 per 100 PY) of patients treated with JESDUVROQ and 5.2% (2.3 per 100 PY) of patients treated with rhEPO. No evidence of increased carcinogenicity was observed in animal studies.
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10%) are hypertension, thrombotic vascular events, and abdominal pain.
DRUG INTERACTIONS
Moderate CYP2C8 Inhibitors: Concomitant administration of moderate CYP2C8 inhibitors (e.g., clopidogrel) increases daprodustat exposure. Reduce the starting dose of JESDUVROQ by half when initiating treatment in patients on a moderate CYP2C8 inhibitor except in patients whose starting dose is already 1 mg. Monitor hemoglobin and adjust the dose of JESDUVROQ when initiating or stopping therapy with a moderate CYP2C8 inhibitor during treatment with JESDUVROQ.
CYP2C8 Inducers: CYP2C8 inducers (e.g., rifampin) may decrease daprodustat exposure, which may result in loss of efficacy. Monitor hemoglobin and adjust the dose of JESDUVROQ when initiating or stopping therapy with CYP2C8 inducers during treatment with JESDUVROQ.
USE IN SPECIFIC POPULATIONS
Pregnancy: JESDUVROQ may cause fetal harm. CKD is associated with maternal and fetal risks. Advise pregnant women of the potential risk to the fetus.
Lactation: Given the serious adverse reactions seen in adults treated with JESDUVROQ, such as thrombotic vascular events, advise patients not to breastfeed during treatment with JESDUVROQ, and for one week after the final dose.
Hepatic Impairment: Reduce the starting dose of JESDUVROQ by half in patients with moderate hepatic impairment (Child-Pugh Class B) except in patients whose starting dose is already 1 mg. JESDUVROQ has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and is not recommended in these patients.
DRUG ABUSE AND DEPENDENCE
Abuse: Abuse of JESDUVROQ may be seen in athletes for the effects on erythropoiesis. There are no data on the abuse of JESDUVROQ in humans. Misuse of drugs that increase erythropoiesis, such as JESDUVROQ, by healthy persons may lead to polycythemia, which may be associated with life-threatening cardiovascular complications.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.